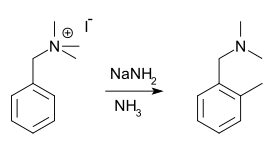
The Sommelet–Hauser rearrangement is a rearrangement reaction of certain benzyl quaternary ammonium salts. The reagent is sodium amide or another alkali metal amide and the reaction product a N,N-dialkylbenzylamine with a new alkyl group in the aromatic ortho position.
Reaction mechanism:
The Stevens rearrangement is a competing reaction.

No comments:
Post a Comment