Antipyretics are substances that reduce fever.
Antipyretics cause the hypothalamus to override a
prostaglandin-induced increase in temperature. The body then works to lower the
temperature, which results in a reduction in fever.
Ibuprofen and
aspirin, which are non-steroidal anti-inflammatory drugs (NSAIDs) used
primarily as analgesics , and antipyretic .
Example:
1.Paracetamol (C8H9NO2)
It is also known as acetaminophen and APAP, is a medication
used to treat pain and fever. It is typically used for mild to moderate pain
relief.
It is also used for
severe pain, such as cancer pain and pain after surgery.
It is typically used either by mouth or rectally, but is
also available by injection into a vein.Effects last between
2 to 4 hours.
It is generally safe at recommended doses.
The recommended maximum daily dose for an adult is 3 or 4 grams.
Higher doses may lead
to toxicity, including liver failure, serious skin rashes may rarely occur.
It appears to be safe during pregnancy and when
breastfeeding.
In those with liver
disease, it may still be used, but in lower doses. It is classified as a mild
analgesic.
It does not have significant anti-inflammatory activity.
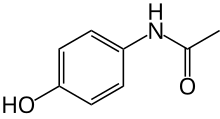
(structure)
Medicinal uses:
It is used for
reducing fever in people of all ages.
It is used to treat
fever in children only if their temperature is higher than 38.5 °C
It is used for the
relief of mild to moderate pain.
It is used for people
with arthritis pain of the hip, hand and dental pain.
Adverse Effect:
Liver damages, asthma, diarrhea, vomiting and abdominal pain.
..................................
Source: Wikipedia