The Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst.
The net reaction can also be described as follows:
[PdCl4]2 − + C2H4 + H2O → CH3CHO + Pd + 2 HCl + 2 Cl−
This conversion is followed by reactions that regenerate the Pd(II) catalyst:
Pd + 2 CuCl2 + 2 Cl − → [PdCl4]2− + 2 CuCl
2 CuCl + ½ O2 + 2 HCl → 2 CuCl2 + H2O
The net reaction can also be described as follows:
[PdCl4]2 − + C2H4 + H2O → CH3CHO + Pd + 2 HCl + 2 Cl−
This conversion is followed by reactions that regenerate the Pd(II) catalyst:
Pd + 2 CuCl2 + 2 Cl − → [PdCl4]2− + 2 CuCl
2 CuCl + ½ O2 + 2 HCl → 2 CuCl2 + H2O
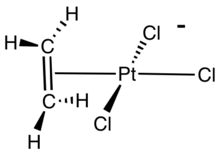
Zeise's salt formation:
This compound is commercially available as a hydrate. The hydrate is commonly prepared from K2[PtCl4] and ethylene in the presence of a catalytic amount of SnCl2.

The alkene C=C bond is approximately perpendicular to the PtCl3 plane. In Zeise's salt and related compounds, the alkene rotates about the metal-alkene bond with a modest activation energy. Analysis of the barrier heights indicates that the π-bonding between most metals and the alkene is weaker than the σ-bonding. In Zeise's anion, this rotational barrier has not been assessed.

No comments:
Post a Comment