Hofmann rearrangement, also known as Hofmann degradation and not to be confused with Hofmann elimination, is the reaction of a primary amide with a halogen (chlorine or bromine) in strongly basic (sodium or potassium hydroxide) aqueous medium, which converts the amide to a primary amine. For example:
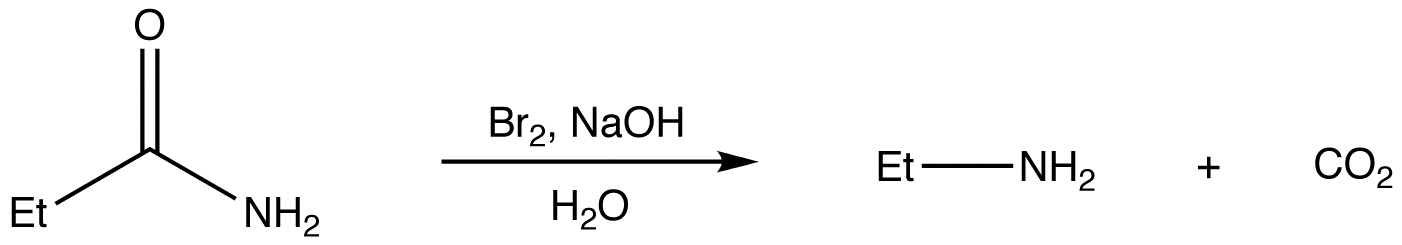
Mechanism:
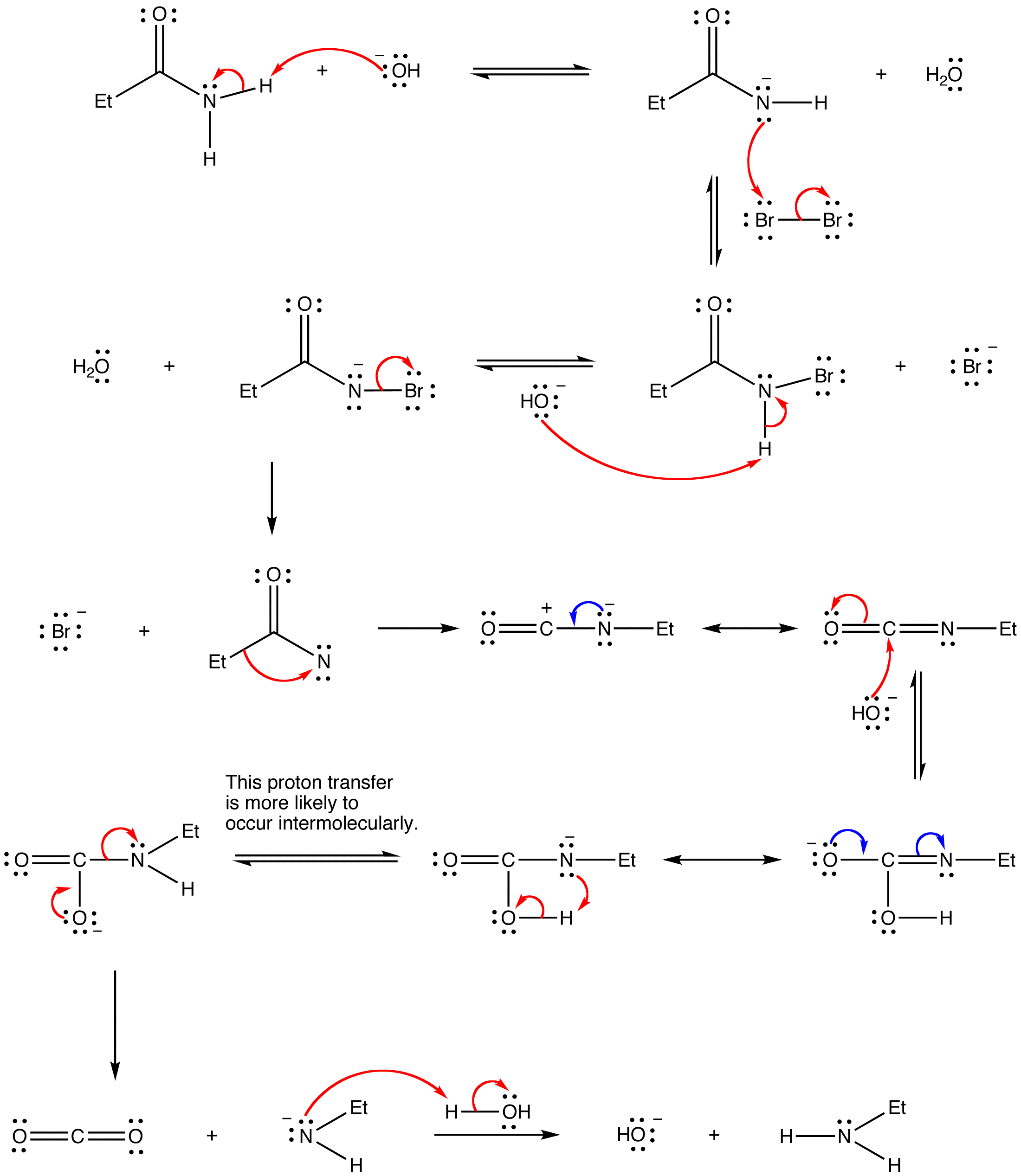
Applications:
Aliphatic & aromatic amides are converted into aliphatic and aromatic amines, respectively
In the preparations of anthranilic acid from phthalimide
Nicotinic acid is converted into 3-Aminopyridine
The symmetrical structure of α-phenyl propanamide does not change after Hofmann reaction.
No comments:
Post a Comment