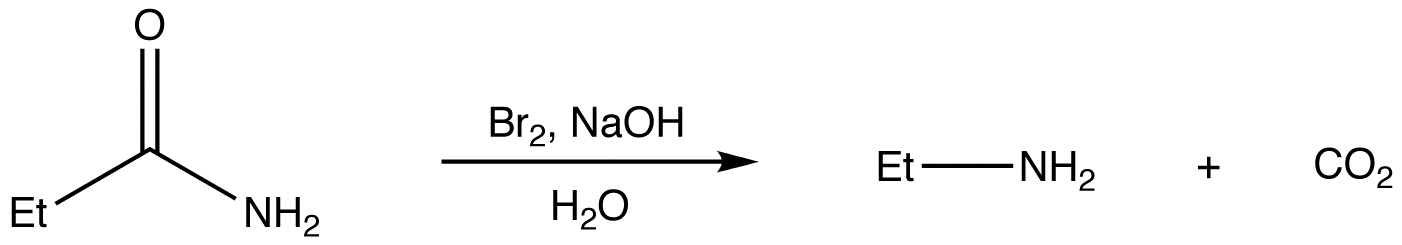
This is a tabular arrangement of the chemical elements.
Elements are arranged by atomic number and electronic configurations.
Periodic table was developed by Dmitri Mendeleev in 1869.
The periodic table can be used to derive relationships between the various element properties, and also to predict chemical properties and behaviours.
The seven rows of the table, called periods(A period in the periodic table is a horizontal row. All elements in a row have the same number of electron shells.)
Generally metals on the left and non-metals on the right.
The columns, called groups( is a column of elements in the periodic table of the chemical elements), contain elements with similar chemical behaviours.
The modern periodic table now provides a useful framework for analyzing chemical reactions, and used in nuclear physics and other sciences.
The elements from atomic numbers 1 (hydrogen) to 118 (oganesson) have been discovered or synthesized, completing seven full rows of the periodic table.
The first 98 elements all occur naturally, though some are found only in trace amounts and a few were discovered in nature.
Elements 99 to 118 have only been synthesized in laboratories or nuclear reactors
Each chemical element has a unique atomic number (Z) representing the number of protons in its nucleus.
Most elements have differing numbers of neutrons among different atoms, with these variants being referred to as isotopes.
For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent have seven neutrons, and a very small fraction have eight neutrons.
Isotopes are never separated in the periodic table; they are always grouped together under a single element.
In the standard periodic table, the elements are listed in order of increasing atomic number Z (the number of protons in the nucleus of an atom).
Lanthanides and actinides:
The lanthanide and actinide series make up the inner
transition metals.
The lanthanide series includes elements 58 to 71, which fill
their 4f sublevel progressively.
The actinides are elements 89 to 103 and fill their 5f sublevel
progressively.
Actinides are typical metals and have properties of both the
d-block and the f-block elements, but they are also radioactive.
Lanthanides have different chemistry from transition metals
because their 4f orbitals are shielded from the atom’s environment.
The 14 elements (numbers 58 to 71) of the lanthanide series
are also known as the rare earth elements. Most lanthanides are formed when
uranium and plutonium undergo nuclear reactions. Atomic bombs charged with
plutonium (actinoid) were used in World War II